The Biodiesel Chemistry Behind
Transesterification
Search this site!
A Briefing on Biodiesel Chemistry:Here's how vegetable oil, a little catalyst, and some methanol react to form biodiesel:
Fats and oils are composed of triglycerides (a type of ester).
A triglyceride is an alcohol (glycerin) that is bonded to three fatty acids (long hydrocarbon chains). Click here to go into greater depth on
triglycerides
(opens in new window).
In order to make biodiesel, the triglycerides must be broken, a few ingredients added, and new esters formed.
The process that turns the triglycerides into glycerin and fatty-acid-methyl-esters (biodiesel) is called trans- esterification.
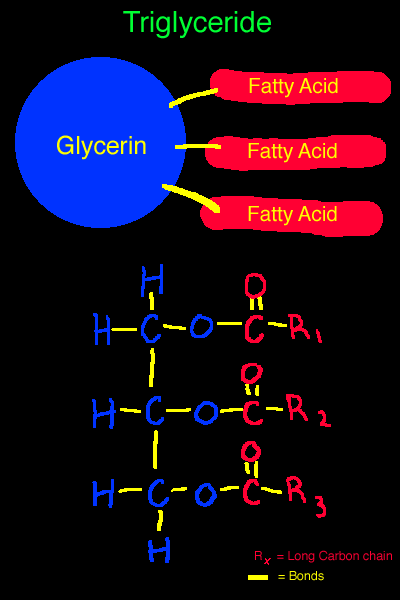 A Triglyceride Molecule:
A Triglyceride Molecule:
In reference to this visual aid...
Notice the glycerin is attached by single bonds to three fatty acids.
On the lower half of the diagram you can see the chemical composition of the same triglyceride.
The glycerin portion is represented in blue, the fatty acid portion in red.
© 2009 www.answers-to-your-biodiesel-questions.com
This image may be copied for use as long as credit is given
and a link back to this page is posted. It may not be altered
in any way.
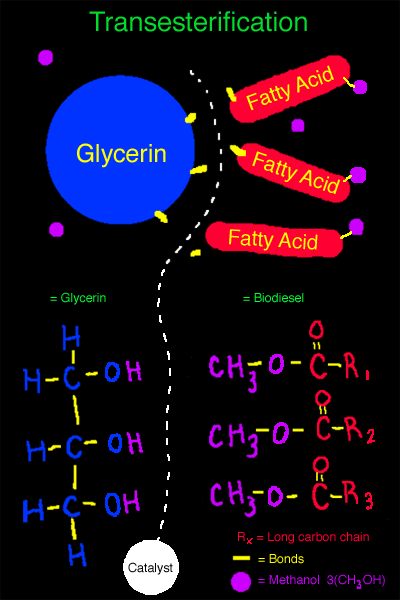 The Chemistry of Transesterification:
The Chemistry of Transesterification:
This second diagram shows what happens during trans- esterification.
There are now methanol molecules present, and a catalyst has been introduced.
The catalyst has broken the bonds between the glycerin and the three fatty acids.
The methanol has bonded to the fatty acids and has formed biodiesel and also a crude glycerin has been formed.
On the lower half of the diagram you can see what has happened chemically.
© 2009 www.answers-to-your-biodiesel-questions.com
This image may be copied for use as long as credit is given
and a link back to this page is posted. It may not be altered
in any way.
Applying Transesterification to Biodiesel Production:
When making biodiesel, to begin the process of trans- esterification, the solution containing the dissolved catalyst and methanol is added to the warmed oil (the oil is warmed in order to speed up the process of trans- esterification). At this point the triglycerides are being broken, and the methanol is bonding, producing biodiesel and crude glycerin. The process takes between 4 and 8 hours when the solution is at 122°F (50°C).
Becoming familiar with terms such as 'triglyceride,' 'esterification,' and 'catalyst' is essential to understanding biodiesel chemistry.
Now have your own
biodiesel chemistry session and make your first 1-liter batch!
Return from 'Biodiesel Chemistry' to 'Biodiesel FAQ'
Biodiesel Homepage